Bioresorbable Medical Devices: State of Art
October is always a busy and fun time of year. Football is mid-season, Halloween is close at hand, and we start planning for the next year at Poly-Med. It’s also the perfect time to step back from the daily task list and think about progress over the last year at Poly-Med and also among medical device innovation overall. And, through this process, I am always honored to be a part of this amazing industry.
We’ve seen some of the largest developments in our field in 2016, such as FDA approval of the Abbott ABSORB® stent which is the highest profile bioresorbable product in recent memory. As in many cases, the use of bioresorbable materials to replace permanent implants is used as a technique to reduce long term complications. Dr. Gregg Stone, chair of the ABSORB® clinical program, reflected on the potential upside of bioresorbable stents compared to permanent metal versions including no residual implant which may reduce future blockages and non-interference for future procedures. When considering potential impact of the product and associated clinical trials, Dr. Stone said “if those trials are positive, then I think bioresorbable technology will ultimately be used in the majority of patients..” ¹
Additive manufacturing is also a trending topic, with big investments from a variety of companies. While the use of bioresorbable polymers in 3D printing is limited, it has already saved lives. Polycaprolactone scaffolds have been printed into tracheal splints, custom bone scaffolds have been prepared for reconstruction after significant orthopedic injury, and creating scaffolding with patient-specific structures is an exciting reality. Advanced bioresorbable materials, like those developed at Poly-Med which are specifically tailored to these applications, is supporting continued development of improved techniques for better patient care in this area.
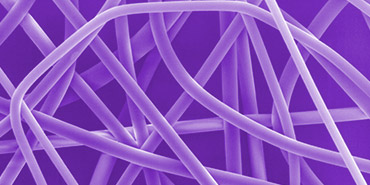
Electrospinning as a process has been known for more than 80 years, though only recently has it advanced to manufacturing feasibility. Significant products, such as the Xeltis bioabsorbable valve² and the Acera Surgical bioabsorbable dura substitute³, both of which are significant because they create a scaffold for native tissue regrowth and highlight the potential impact of electrospinning technology. Over the last year, Poly-Med was able to support several medical device development opportunities utilizing electrospinning, and has made significant advancement in scale and support for new and developing products.
The transition of bioresorbables into mainstream device development confirms the need for enhanced materials, specialty processing, and design support. So as I reflect about the last year within Poly-Med, it has been filled with foundational growth to support this type of development. We completed manufacturing transfer for a bioresorbable electrospun product which recently received market clearance, implemented scaled solutions to support growth in polymer and textile products, and are preparing new material offerings for advanced product design. As it has been throughout my 15 years at Poly-Med, it is fun and exciting to be a part of these innovative, impactful products that can improve patient care and reduce long term risk.
Scott Taylor, Ph.D.
Chief Technology Officer
Poly-Med, Inc.
¹ Reference
² Reference
³ Reference