Filling the Gap: Tissue Adhesives & Surgical Sealants
Acute wounds have long represented a major focus for healthcare professionals and clinicians. While traditional wound care strategies have revolved around the use of sutures, staples, and tack-type devices, significant development has focused on the expansion of topical tissue adhesives and surgical sealants.
Overall, the global market for wound care has reached over $10.8 billion (2014) with forecasted growth of ~$14 billion in 2018.1
Current product offerings within the tissue adhesive and surgical sealant field strive to meet:
- • long-lasting microbial protection
- • integration into wound-site
- • sufficient tensile strength
- • flexibility and compliance on curved surfaces (i.e. elbows, knees, etc.)
These materials are typically synthetic or naturally-derived and are meant to restore compromised tissue boundaries limiting infection while allowing healing to occur. Based on the demonstrated efficacy of adhesives and sealants, their use independent of other traditional closure technologies (i.e. sutures) has been rapidly increasing with exceptional growth (CAGR +15%)1.
Poly-Med, a world leader and innovator in bioresorbable polymers, is active in the tissue adhesive and surgical sealant space and has been for more than twenty (20) years. Within this time, the Poly-Med team has worked to address common problems present in topical wound care products including limited viscosity, low compliance (i.e. brittleness), and poor integration at the wound site. To overcome these limitations, Poly-Med has developed a range of bioresorbable modifiers that can be incorporated directly into a variety of wound closure systems.
Poly-Med’s patented chemistry can be directly added into well-known adhesive systems (i.e. cyanoacrylates) currently on the market. From our extensive polymer catalogue, Caproprene™ and Strataprene® polymer families provide a full range of potential additives that can offer customizable viscosity with enhanced compliance.
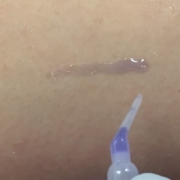
Strataprene® is a fully traceable, medical grade polymer and offers customizable molecular weight while also exhibiting elastic-like properties (able to undergo 1000% strain prior to breaking2). In addition to being used as a modifier for tissue adhesive systems, Strataprene® 3534 can be used as a spray bandage or film that can be directly applied to the patient’s skin in the clinical setting. Strataprene®’s enhanced solubility allows use with a host of benign solvents, making it readily dissolvable and able to integrate with a patient’s skin. Strataprene® can also readily combined with a host of active pharmaceutical ingredient’s and can provide a variety of release profiles for extended wound protection and healing.2
If you are working on an advanced formulation for a tissue adhesive or surgical sealant,
Contact us to learn how your project can benefit from Poly-Med’s innovative wound care technologies.
Seth McCullen, Ph.D.
Manager, Business Development, Poly-Med, Inc.
1 L. MedMarket Diligence, Report #S190, Worldwide Surgical Sealants, Glues, Wound Closure and Anti-adhesion Markets, 2010–2017.
2 Internal data on file at Poly-Med, Inc.